Tai Xi , Li Deliang , Li Haitao , Hu Qiufen 1,2Yang Guangyu 2*, YinJiayuan
(1Department of Chemistry, Yuxi Teacher’s College, Yuxi, 653100; Department of Chemistry, Yunnan University, Kunming, 650091,China)
Received Dec. 23, 2003; Supported by Natural Science Foundation of Yunnan Province (0111143) and Yuxi Teacher’sCollege (02476).
Abstract A new method for the simultaneous determination of four heavy metal ions, nickel, lead, cadmium and mercury ions as metal-tetra-(2-aminophenyl)-porphyrin (APP) chelates was developed using reversed-phase high performance liquid chromatography (RP-HPLC) equipped with a photodiode array detector and combined with an on-line enrichment technique. The detection limits (S/N=3) of nickel, lead, cadmium and mercury are 3.5 ng/L, 2.5 ng/L, 3.0 ng/L and 3.0 ng/L, respectively. This method has been applied to the determination of nickel, lead, cadmium and mercury ions in water with good results.
The RP-HPLC techniques with pre-column derivatization have been proved to be a favorable and reliable technique for the determination of trace amount of metal ions. Many kinds of reagents have been examined as pre-column derivatization regents, and several review articles have appeared on this approach [1-4]. Among the various kinds of reagents, porphyrin ligands are useful because of its high molar absorptivity and high stability [5-7]. In this paper, we report the preconcentration and separation of Ni-TAPP, Pb-TAPP, Hg-TAPP and Cd-TAPP chelates on a Waters XterraTMRP18 column (pH 1-12) with a valve switching HPLC system equipped with a photodiode array detector. By this system, the injection of a large volume of sample was possible and the Ni-TAPP, Pb-TAPP, Hg-TAPP and Cd-TAPP chelates were successfully separated. This method has been applied to the determinationg/L (ppb) level of nickel, lead, cadmium and mercury ions in water with good results.
1. EXPERIMENTAL PROCEDURES
1.1 Apparatus
The on-line enrichment system used is shown in Fig 1, including a Waters 2690 Alliance quadripump, Waters 515 pump, Waters 996 photodiode array detector, six-port switching valve, large volume injector ( containing a 5.0 ml sample), enrichment column [Waters XterraTM RP18 (5 m m, 3.9×20 mm)] and analytical column [Waters Xterra TM RP18 (5m, 3.9×150 mm)]. The pH value was determined with a Beckman-200 pH meter.
1.2 Chemicals
All of the solutions were prepared with ultra-pure water obtained from a Milli-Q50 SP Reagent Water System (Millipore Corporation, USA). Nickel(II), lead(II), cadmium(II) and mercury(II) standard solution of 1.0 mg/ml was obtained from the Chinese Standards Center, and a working solution of 0.2g/ml was prepared by diluting this standard solution. HPLC grade methanol and THF (Fisher Corporation, USA), pyrrolidine-acetic acid buffer solution, 0.5 mol/L, pH=10, and TritonX-100 solution, 1.0% (v/v) were used. TAPP was synthesized in our laboratory as in the literature [8], and was dissolved with THF to make a 1.0×10-4 mol/L of solution. Mobile phase A: 0.05 mol/L pH=10 pyrrolidine-acetic acid buffer solution. Mobile phase B: methanol (containing 0.05 mol/L pH=10 pyrrolidine-acetic acid buffer salt). Mobile phase C: acetone (containing 0.05 mol/L pH=10 pyrrolidine-acetic acid buffer salt). All other reagents used were of analytical reagent-grade. The glass and Teflon ware used were soaked in 5% of nitric acid overnight, and then thoroughly washed with pure water.
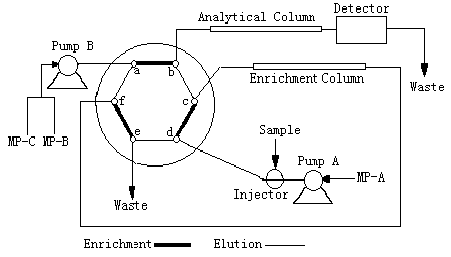
Fig. 1 On-line enrichment system using the valve-switching technique
Pump A, Waters 515 Pump. Pump B, Waters 2690 Alliance quadripump. Injector can contain 5 ml of sample. Six-port switching valve (Waters Corporation). Enrichment Column, Waters XterraTM RP18 (5m , 3.9×20). Analytical column, Waters XterraTM RP18 (5m , 3.9×150). Detector, Waters 996 photodiode array detector. MP A, 0.05 mol/L pH=10 pyrrolidine-acetic acid buffer solution. MP B, Methanol (containing 0.05 mol/L of pH=10 pyrrolidine-acetic acid buffer salt). MP C, acetone (containing 0.05 mol/L of pH=10 pyrrolidine-acetic acid buffer salt).
1.3 Standard Procedure
0-15 ml of 0.2g/ml standard or sample solution was transferred into a 25 ml volumetric flask, for which, 5.0 ml of 1.0×10-4 mol/L TAPP THF solution, 2 ml of Triton X-100 solution and 3 ml of 0.5 mol/L pyrrolidine-acetic acid buffer solution (pH=10) were added. The solution was diluted to the volume with water and mixed well. The mixture was heated in a boiling water bath for 15 min. After cooling, the solution was diluted to the volume with THF for subsequent analysis. 5.0 ml of solution was introduced into the injector and sent to the enrichment column with mobile phase A at a flow rate of 2.0 ml/min. After the enrichment had been finished, by switching the valve of the six-port switching valve, the metal-TAPP chelates absorbed onto the foreside of the enrichment column, were eluted by mobile phase B and C at the flow rate of 1.0 ml/min in reverse direction and traveled towards the analytical column and separated on it. A tridimensional (X axis: retention time, Y axis: wavelength, Z axis: absorbance) chromatogram was recorded from 350- 600 nm with a photodiode array detector and the chromatogram of 450 nm is shown in Fig 2. In the course of separation, the composition of mobile phase is: 0 min (100% B and 0% C),10 min (90 % B and 10% C) in linear ramp. Each metal-TAPP chelate was detected at its maximum absorption wavelength.

Fig.2 Chromatogram of standard sample and water sample:a) Standard sample,b) Water samples
Injection volume 5ml. The concentration of Ni(II), Hg(II), Pb(II), Cd(II) is 10 m g/L in standard sample. Detection wavelength is 450 nm. Other conditions as in standard procedure
2. RESULTS AND DISCUSSION
2.1 Precolumn Derivation
In a weak alkaline medium of pH 8.5-12, Ni(II), Hg(II), Pb(II) and Cd(II) can form stable and colored chelates with TAPP, so a 0.5 mol/L of pH=10 pyrrolidine-acetic acid buffer solution was recommended to control pH.
It was found that 0.5 ml of 1.0×10-4 mol/L T2APP THF solution was sufficient to complex 3.0 m g of Ni(II), Hg(II), Pb(II) and Cd(II), respectively. But in real samples, the foreign ions, such as Mg2+, Cu2+, Pd2+, Ru2+, Bi3+, Co2+, Fe3+, Mn2+, Sn(IV), Bi3+, Zn2+ and the like, form complexes with TAPP and consume reagents. So the amount of TAPP must be in excess. In this experiment, A 5.0 ml of 1.0×10-4 mol/L TAPP solution was recommended.
The reaction of Ni(II), Hg(II), Pb(II) and Cd(II) with TAPP was slow at room temperature. Heating can accelerate the reaction. The reaction was completed by heating in a boiling water bath for 15 min and the complex was stable for at least 4 h after cooling, so that heating 15 min in a boiling water bath was selected.
2.2 On-Line Enrichment
Ni-TAPP, Hg-TAPP, Pb-TAPP and Cd-TAPP chelates are stable in weak alkaline medium. To avoid the chelates decomposing during the elution, a 0.05 mol/L of pH=10 pyrrolidine-acetic acid buffer solution (mobile phase A) was selected as mobile phase to send the chelates to the enrichment column and a Waters XterraTM RP18 chromatographic column (5m, 3.9×20 mm) with pH range 1- 12 was selected as enrichment column. Experiments showed that the volume of a 5 ml sample injected was sensitive enough to determine Ni(II), Hg(II), Pb(II) and Cd(II) in samples, so a 5 ml sample injection was recommended.
2.3 Spectrophotometric Properties
From a tridimensional chromatogram recorded by photodiode array detector, the absorption spectrum of metal-TAPP chelates was obtained. The maximum absorption wavelengths of Ni-TAPP, Hg-TAPP, Pb-TAPP and Cd-TAPP are 434 nm, 452 nm, 468 nm and 440 nm, respectively. To get maximum sensitivity, each metal-TAPP chelate was monitored at its maximum absorption wavelength.

Fig.3 The effect of mobile phase pH on peak area
2.4 Chromatographic Separation
The Ni-TAPP, Hg-TAPP, Pb-TAPP and Cd-TAPP chelates were stable in weak alkaline medium. So the effect of mobile phase pH on chromatographic peak was studied (Fig 3). Experiments showed that the pH of mobile phase within 8.8- 11.5 can avoid the chelates decomposing and get a maximum and constant peak area. So two weak alkaline solutions, mobile phase B: methanol (containing 0.05 mol/L of pyrrolidine-acetic acid buffer salt (pH=10.0)), and mobile phase C: acetone (containing 0.05 mol/L of pyrrolidine-acetic acid buffer salt (pH=10.0)) were recommended. Since the common reserved phase chromatographic column could not remain stable at pH 10, a Waters XterraTM RP18 chromatographic column (5m, 3.9×150) was selected as analytical column in this experiment. XterraTM RP18 columns have good stability in pH 1- 12. The relative proportions of mobile phase B and C were varied to effect the separation. Experiment showed that gradient elution achieved good results. The proper composition of mobile phase during the gradient elution was selected as follows: 0 min (100% of B and 0%of C),10 min (90% of B and 10% of C) in linear ramp.
2.5 Calibration Graphs
Under optimum conditions, regression equations of metal-TAPP chelates were established based on the standard samples injected and their peak areas. Limits of detection are calculated by the ratio of signal to noise (S/N=3). The reproducibility of this method was also examined for 10g/L of Ni(II), Pb(II), Cd(II) and Hg(II). The results are shown in Table 1.
Table1 Regression Equation, Coefficient and Detection Limit
| Components | Regression Equation | Linearity Range(g/L) | Coefficient | Detect limit (ng/L) | RSD%(n=10) |
| Ni-TAPP | A=1.75×10 C+122 | 0.01~ 120 | r=0.9992 | 3.5 | 2.1 |
| Cd-TAPP | A=2.83×10 C+135 | 0.01~ 120 | r=0.9991 | 2.5 | 2.3 |
| Pb-TAPP | A=1.74×10 C+212 | 0.01~ 120 | r=0.9995 | 3.0 | 2.2 |
| Hg-TAPP | A=1.86×10 C-208 | 0.01~ 120 | r=0.9994 | 3.0 | 2.4 |
2.6 Interference
Under the pre-column derivatization condition, the foreign ions of Mg2+,Cu2+,Pd2+,Bi3+,Co2+,Fe3+,Mn2+,Sn(IV), Zn2+,Pt2+, Ba2+ and Ag react with TAPP to form color chelates. To examine the selectivity of this method, the interference of foreign ions was investigated. When 5.0 ml of 1.0×10-4 mol/L TAPP was used, with 10 m g/L of Ni(II), Pb(II), Cd(II) and Hg(II), respectively, the amount tolerated with an error of ± 5% is shown in Table 2. The results showed that most foreign ions do not interfere with the determination. This method is highly selective.
Table 2 Tolerance amount of foreign ions (error of ± 5%)
| Tolerance amount(g/L) of foreign ion for 10g/L of Sn(IV), Ni(II), Cd(II), Pb(II) and Hg(II) | |||||||||||
| Ag(I) | Mg2+ | Cu2+ | Pd2+ | Bi3+ | Co2+ | Fe3+ | Mn2+ | Zn2+ | Pt2+ | Ba2+ | |
| Ni(II) | 500 | 25000 | 3000 | 1000 | 1500 | 1000 | 3000 | 3500 | 1500 | 1000 | 1000 |
| Cd(II) | 1500 | 10000 | 2500 | 500 | 1000 | 1500 | 2500 | 4000 | 2000 | 2000 | 2000 |
| Pb(II) | 1000 | 15000 | 1000 | 800 | 1500 | 1500 | 1500 | 5000 | 1500 | 500 | 2000 |
| Hg(II) | 500 | 10000 | 1500 | 1000 | 1000 | 1000 | 3000 | 3000 | 1000 | 1000 | 1500 |
2.7 Application to Water Sample
For the fresh water (tap water, river water and lake water) the water sample was analyzed according to the general procedure. The results (deducted the reagents blank) were shown in Table 3, together with the results of a recovery test by added 0.2g of Ni, Pb, Cd and Hg in water sample and diluted to 50 ml of final solution. For plant effluents, the sample was digested as in the literature [6] and analyzed according to the general procedure. The results (deducted the reagents blank) were shown in Table 3 too, together with the results of a recovery test by added 0.2g of Ni, Pb, Cd and Hg in water sample and diluted to 50 ml of final solution. A standard method using atomic absorption spectrometry had also been used as reference method. The results are shown in Table 4.
Table 3 Determination results (g/L) of the water sample with this method
| Components | Samples (g/L) | RSD%(n=5) | Recovery%(n=5) | |||
| River water | Lake water | Plant effluent | Tap water | |||
| Ni | 26.2 | 15.8 | 44.2 | 16.2 | 2.3 | 97-103 |
| Cd | 5.46 | 11.2 | 16.7 | 2.22 | 2.2 | 97-104 |
| Pb | 12.5 | 8.65 | 22.8 | 6.58 | 2.1 | 96-102 |
| Hg | 2.87 | 5.62 | 12.5 | 1.7 | 98-105 |
Table 4Determination results (m g/L) of the water sample with reference method
| Components | Samples (g/L) | RSD%(n=5) | Recovery%(n=5) | |||
| River water | Lake water | Plant effluent | Tap water | |||
| Ni | 28.2 | 16.5 | 48.5 | 15.2 | 3.2 | 95-105 |
| Cd | 5.96 | 11.8 | 15.2 | 2.13 | 3.5 | 96-108 |
| Pb | 11.8 | 8.76 | 24.4 | 6.76 | 3.0 | 91-102 |
| Hg | 2.81 | 5.41 | 13.5 | 3.2 | 95-106 |
3. CONCLUSION
The proposed method has the following advantages: (1) Four toxic heavy metal ions, Ni(II), Pb(II), Cd(II) and Hg(II) were successfully separated in the proposed method using a buffer solution of pH 10 as mobile phase on Waters XterraTM RP18 Column. (2) By an on-line enrichment system, a large volume of sample (5 ml) can be injected. (3) With a photodiode array detector, each metal-chelate can be monitored at its maximum absorption wavelength to achieve maximum sensitivity. In a word, for the determination of nickel, lead, cadmium and mercury ions inwater, this method is highly sensitive and highly selective.
