Lu Yunkai, Lu Guodong, Qin Xinying, Liu Yue
(College of Chemistry and Environmental Science, Hebei University, Key Laboratory of Analytical Science and Technology of Hebei Province, Baoding 071002, China)
Abstract:A new diamine functionalized glass fibre sorbent was prepared for preconcentration and separation of toxic metal ions from aqueous solution by loading tetraethoxysilicate (TEOS) and N-(2-aminoethyl)-3-aminopropyltrimethoxysilane (AAPTS) on glass fibre through a sol-gel process. The new sorbent provided good acid-base stability, fast adsorption and desorption kinetics (no loss of adsorption and desorption efficiencies at flow rates up to at least 11.6 mL min-1). The dynamic capacity for 2 mg L-1 Cd(II) and 1.5 mg L-1 Cu(II) at a loading flow rate of 5.6 mL min-1 was 0.146 and 0.150 mmol g-1, respectively. The applicability of the developed sorbent for on-line preconcentration and separation of trace Cd(II) and Cu(II) in environmental and biological materials was demonstrated by a hyphenated technique of flow injection microcolumn coupled on-line with flame atomic absorption spectrometry. With a sample loading flow rate of 9.6 mL min-1 for 30 s preconcentration, the enhancement factors of 124 for Cd(II) and 61 for Cu(II), and the detection limits (3s ) of 14 ng L-1 for Cd(II) and 31 ng L-1 for Cu(II) were achieved at a sampling frequency of 80 h-1. The precision (RSD) for 11 replicate preconcentrations of 4 m g L-1 Cd(II) and 10 m g L-1 Cu(II) was 0.8% and 0.5%, respectively. The new sorbent also offered good linearity (g >0.9995) for on-line preconcentration of trace Cd(II) and Cu(II).
1. INTRODUCTION
Development of new sorbents for separation and preconcentration of toxic metal ions in complicated matrices plays an important role in environmental analysis and clean-up.1-5 Solid-phase extraction is a very promising technique for separation and preconcentration, its development is based on designing new solid sorbents with high sorption capacity, selectivity and suitability for on-line applications.
One of the most widely used supporting materials for preparing selective sorbents is inorganic solid support, such as silica gel and controlled pore glass.6, 7 Recently, glass fibre have gained growing interest as an important support for the immobilization of biological proteins, which has been found wide application in both analytical and preparative systems.8, 9 Glass fibre, a flexile, widely available material, holds many remarkable advantages compared with other support, including low cost (unlike controlled porosity glasses), excellent mechanical strength, durability, thermal stability, good kinetic characteristics, and its easy modification with various functional groups.
Surface modification of glass fibre to obtain sorbents with desired functional groups is made possible via the SiOH groups. However, the inherent limitation in the availability of hydroxyl groups on the surface of glass fibre leads to small metal adsorption capacity. To increase the adsorption capacity and the immobilization layer stability, a sol-gel method was used in modifying glass fibre, and then the functional groups were introduced onto the surface. N-(2-aminoethyl)-3- aminopropyltrimethoxysilane (AAPTS) was used to reinforce fibres.10 Immobilization of AAPTS on glass fibre may be easily converted into various chelating groups with the specificity toward metal cations (such as dithiocarbamates11) and the immobilization of biological proteins.
In the present work, a new diamine-functionalized glass fibre sorbent (AAP-GW) was synthesized by loading tetraethoxysilicate (TEOS) and N-(2-aminoethyl)-3-aminopropyltrimethoxysilane (AAPTS) on glass fibre through a sol-gel process, and evaluated for on-line preconcentration and separation of toxic metal ions from aqueous solution using Cd(II) and Cu(II) as model metal ions.
2. EXPERIMENTAL
2.1 Materials and chemicals
All reagents used were of the highest available purity and at least of analytical grade. All metal stock solutions (100 mg L-1) were purchased from National Research Center for Standard Materials (NRCSM, Beijing, China). The working solutions were prepared by series dilution of the stock solutions immediately prior to use. Doubly deionized water (DDW, 18.2 MΩ cm) obtained from a WaterPro water system (Labconco Corporation, Kansas, MO, USA) was used throughout the experiments. Two silane agent, Tetraethoxysilicate (TEOS) and N-(2-aminoethyl)-3- aminopropyltrimethoxysilane (AAPTS, Wuhan University Chemical Factory, Wuhan, China), and glass fibre (WAHLEE Glass Fibre Co., Ltd. Qingdao, China) were used for sorbent preparation.
2.2 Apparatus and conditions
A ModelSolaar S2flame atomic absorption spectrometer (Thermo Elemental Co.) was employed for determination of trace metals. The AAS instrument was controlled by the SOLAARS operation software. Hollow cathode lamps (Beijing Shuguangming Electronic Light Source Instrument, Beijing) were used as the radiation source at 228.8 nm for Cd and 324.8 nm for Cu) with a 0.5-nm slit width. The recommended acetylene flow rates of 1.1 L min-1 were employed. The air flow rates were automatically adjusted to meet the stoichiometric air-acetylene flame conditions.
A Model FIA-3100 flow injection analyzer (Vital Instrumental, Beijing, China) was used to evaluate the applicability of the sorbent for on-line preconcentration and separation of trace Cd(II) and Cu(II). The FIA-3100 consists of two peristaltic pumps and a standard rotary injection valve (8-channel 16-port multifunctional injector). Tygon peristaltic pump tubings were employed to propel the sample and reagent. PTFE tubing with 0.35-mm i.d. was used for all connections. These connections were kept as short as possible to minimize the dead volumes.
2.3 Preparation of Diamine Functionalized Glass Fibre Sorbent
The glass fibre was washed thoroughly with dilute hydrochloric acid, doubly distilled water, boiled in ethanol for 2 h and then dried at 120℃for 12 h. The pretreated glass fibre was stored in a vacuum desiccator for subsequent immobilization.
A sol solution was prepared by dissolving 1 g TEOS in 9 mL of cosolvent of methanol (95% in total solvent) and deionized water (5%) at pH 4.0 (with acetic acid solution) for 20 min. 0.5 g of pretreated glass fibre was soaked in the sol solution for 30 min, isolated from the solution, air-dried, and conditioned at 120℃for 4 h. The conditioned material was soaked in 10 mL of 10 % AAPTS in methanol solution for 4 h, isolated from the solution, and dried under vacuum at 80℃for 12 h. After washing with deionized water, ethanol and deionized water sequentially, the AAPTS immobilized glass fibre (AAP-GW) was neutralized with NaHCO, washed with deionized water again, dried, and stored in a vacuum desiccator.
For comparison, 10% AAPTS in dry toluene solution, in aqueous solution and in methanol solution were used, respectively, in immobilization of AAPTS on glass fibre without TEOS sol-gel pretreatment.
Determination of the static uptake capacity. 0.1 g of the AAP-GW sorbent was equilibrated with 10 mL of the buffer solution containing 2 mmol L-1 of Cd(II) or 2 mmol L-1 of Cu(II) in stoppered plastic vials and these mixtures were stirred for 30 min at room temperature. The filtrates were measured for uptakes of Cd(II) and Cu(II) by a Model Solaar S2 flame atomic absorption spectrometer.
2.4 Determination of dynamic capacity
The dynamic capacity of the AAP-GW sorbent was determined by passing Cd(II) and Cu(II) solution at optimum pH through a cylindrically shaped PTFE microcolumn (3-cm long ´ 2-mm i.d.) packed with 22 mg of the sorbent at a fixed flow rate until the concentration of the metal ions in the effluent was the same as in the influent. Successively fractions of 10 mL effluent portions were collected, in which the metal contents were measured by FAAS.12 The dynamic capacity was calculated according to Wang and Barnes.13
2.5 Procedures for applicability evaluation of the AAP-GW sorbent for on-line separation and preconcentration
A hyphenated technique, namely flow injection (FI) on-line microcolumn preconcentration and separation coupled with flame atomic absorption spectrometry (FAAS) using a cylindrically shaped microcolumn (3-cm long×2-mm i.d.) packed with 22 mg of the AAP-GW sorbent was employed to evaluate the applicability of the AAP-GW sorbent for on-line separation and preconcentration of trace Cd(II) and Cu(II). The FI manifold and its operation sequence for on-line microcolumn preconcentration and separation are shown in Figure 1 and Table 1, respectively.
Table 1. Operational Sequence of the FI On-Line Microcolumn Preconcentration System Coupled with FAAS for Determination of Trace Cd(II) and Cu(II)
| step | function | time/s | pumped medium | flow rate / ml min-1 | palve position | |
| pump 1 | pump 2 | |||||
| sample loading | 30 | sample solution | 9.6 | off | fill | |
| elution | 10 | 0.4 mol L-1 HCl for Cd(II) 0.8 mol L-1 HCl for Cu(II) |
off | 5.6 | injection | |
| column regeneration | 0.2 mol l-1 NH | off | 5.6 | fill |
2.6 Sample digestion
The following certified reference materials (NRCSM) and real samples were analyzed to check the accuracy of the developed FI on-line microcolumn preconcentration FAAS technique using the AAP-GW as sorbent: GBW 07605 (tea), GBW 07601 (human hair), GBW 08607 (river water). 0.3500 g of each sample was mixed with 10 mL concentrated HNO and digested in sealed PFA (Teflon-perfluoralkoxy) vessels using a Model Qwave-2000 microwave digestion system (Questron Co., Canada). All instrumental parameters for the digestion were chosen according to the recommendations of EPA. The clear digest was transferred into a 50-mL calibrated flask and diluted to volume with DDW.
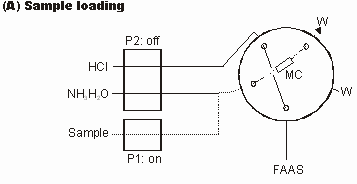

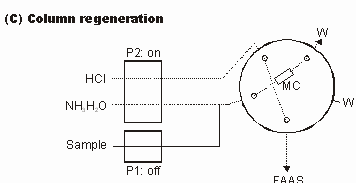
Figure 1FI manifold for evaluation of the AAP-GW sorbent as a new packing for on-line microcolumn preconcentration and separation of trace metal ions. P1 and P2, peristaltic pumps; MC, microcolumn packed with the AAP-GW sorbent; W, waste; FAAS, flame atomic absorption spectrometer; dotted lines are active.
3. RESULTS AND DISCUSSION
Immobilization of chelating groups onto inorganic silica surfaces is usually carried out by reacting chelating agents with silica substrate through hydroxyl groups on the surface in a toluene solution or an aqueous solution. In our preliminary experiments, this method was tried to immobilize diamine group onto glass fibre in toluene, or aqueous, or methanol solution, resulting in low adsorption capacity owing to an inherent limitation in the availability of hydroxyl groups on the surface of glass fibre. Moreover, the sorbents prepared in this way could not suffer higher temperatures due to serious hydrolysis (hydrolysis degree 20-40%, at pH > 8.0) through breaking the bond between the immobilized chelating agent and the surface of glass fibre under alkaline conditions.
To overcome the above problems, we employed a sol-gel process to prepare the diamine functionalized glass fibre sorbent in aqueous solution by loading TEOS and AAPTS on the surface of glass fibre. This method increased the ligand density on the glass fibre surface, metal uptake capacity, and chemical stability of the prepared sorbent. In the following sections, the results for chemical stability, adsorption and desorption characteristics of the sorbent, and the applicability of the sorbent for on-line preconcentration and separation were presented and discussed.
3.1 Immobilization efficiency
The elemental analysis of the AAP-GW for amido suggested a higher free group content of the 1.21 mmol N g-1 (AAP-GW), corresponding to 0.61 mmol (AAP) g-1 (AAP-GW). The static uptake capacity of the AAP-GW sorbent was determined to be 0.236 and 0.242 mmol g-1 for Cd(II) and Cu(II), respectively. It was found that the static capacity of the AAP-GW sorbent after storing for more than 6 months under ambient conditions was still practically unchanged.
3.2 Dynamic capacity of the AAP-GW sorbent
The dynamic capacity describes the operational characteristics of a sorbent in the column operation mode. The theoretical value depends upon the nature of the material and the form of the sorbent, the operational capacity is usually lower than the available capacity, and depends on several experimental factors such as flow rate, temperature, and concentration of the loading solution. Besides, the “breakthrough”of solution from the column defines a dynamic capacity (working capacity), which is lower than the total capacity.12 The dynamic capacity corresponds to the maximum amount of analyte that is retained with minimum leakage of the element from the influent solution. The volume of solution percolated from the breakthrough point to the point of leveling of the loading curve for a given solution flow rate also depends upon the uptake kinetics.12
The dynamic capacity of the sorbent was determined by pumping Cd(II) or Cu(II) solution through a column packed with 22 mg of the AAP-GW sorbent and collecting successive volumes of 10 mL of effluent. The concentration () of metal in each effluent fraction was measured by FAAS, and the ratio of to the concentration of the influent loading solution () was plotted against the effluent volume.
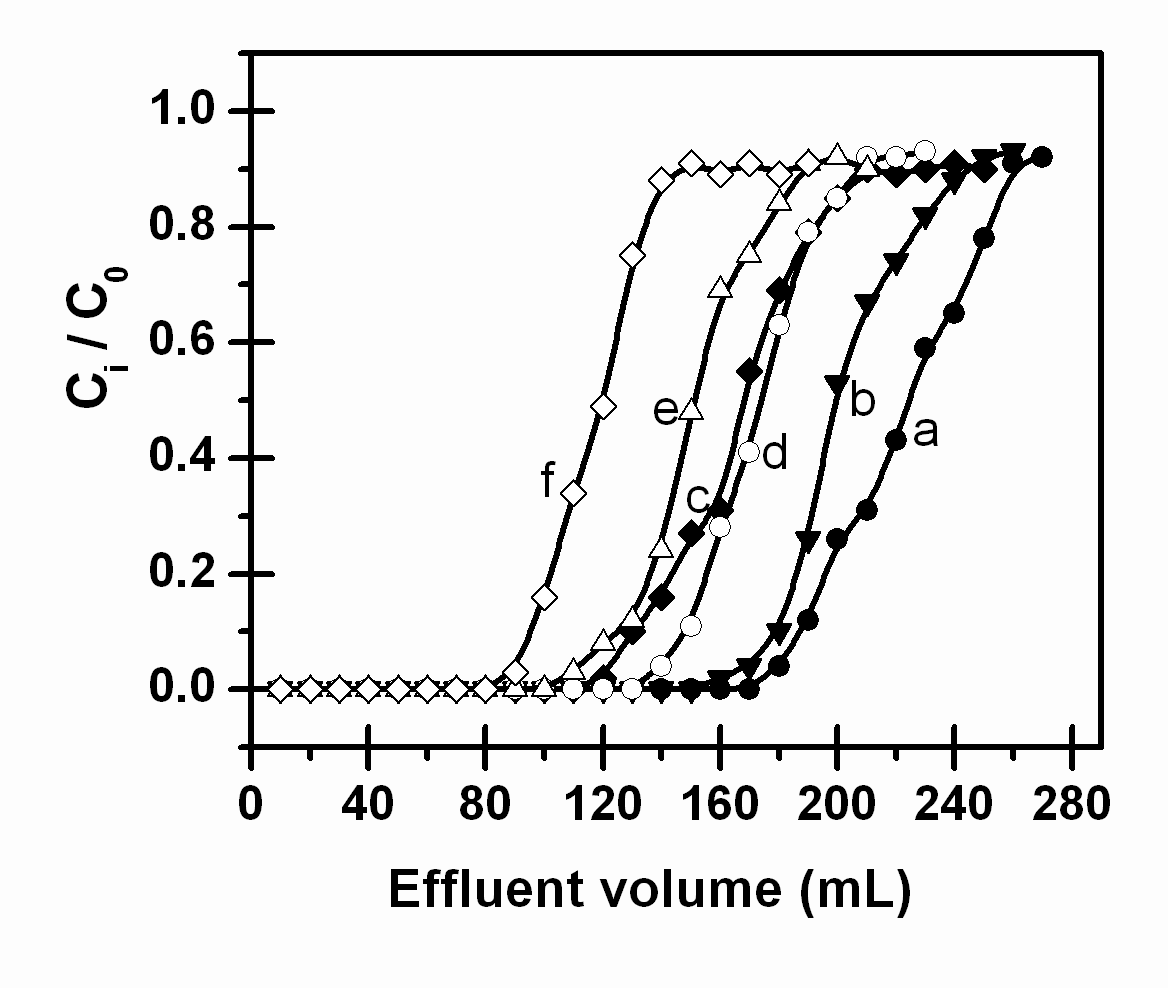
Figure 2. Dynamic capacity curves of the AAP-GW sorbent for 2 mg L-1 Cd(II) (a, b, c)and 1.5 mg L-1 Cu(II) (d, e, f) solution (pH 3.6-4.0) loaded at flow rates of 2.7 (a, d), 5.2 (b, e), and 8 (c, f) mL min-1

Figure 3. Dynamic capacity curves of the AAP-GW sorbent for the different influent concentrations of Cu(II) and Cd(II) solution (pH 3.6-4.0) loaded at a flow rate of 5.2 mL min-1. Cd (mg L-1): (a) 1.5, (b) 2, and (c) 2.5; Cu (mg L-1): (d) 1, (e) 1.5 and (f) 2.
The effect of sample loading flow rate on the breakthrough point was displayed in Figure 2. The results show that, at a given concentration (2 mg L-1 Cd(II) and 1.5 mg L-1 Cu(II)), the dynamic capacity decreased with increasing of sample loading flow rate although the leveling of the curves at / = 1 is not reached. The slope of the ascending portion of the curve also suggests a higher adsorption rate. The effect of the concentration of the metal ion in influent solution on breakthrough point is shown in Figure 3. The results show that at a given sample loading flow rate (5.2 mL min-1), the breakthrough capacity increased as the concentration of the metal ion in influent solution decreased. These results indicate that at low flow rates and low metal concentrations, a large dynamic capacity can be obtained. The dynamic capacities (work capacities) for 2 mg L-1 Cd(II) and 1.5 mg L-1 Cu(II) at a fixed sample loading flow rate (5.2 mL min-1) were calculated to be 0.146 and 0.150 mmol g-1, respectively.
3.3 Evaluation of the AAP-GW Sorbent for On-Line Preconcentration and Separation of Trace Metal Ions
The applicability of the AAP-GW sorbent as a microcolumn packing for on-line preconcentration and separation of trace toxic metal ions was evaluated using a hyphenated technique, FI preconcentration and separation on-line coupled with FAAS. The chemical and flow variables, such as sample acidity, sample loading flow rate and loading time, eluent and its concentration and flow rate, regeneration agents and their concentrations, were optimized to achieve good sensitivity and precision for the retention and elution of Cd(II) and Cu(II).
Sample acidity plays an important role in the complex sorption preconcentration because it affects the complex form between metal ions and functional ligands. The influence of sample acidity on the preconcentration of 4 m g L-1 Cd(II)and 10 m g L-1 Cu(II) were examined at a sample loading rate of 9.6 mL min-1 with 30-s preconcentration. As shown in Figure 4, the progressive decrease in the absorbance at lower pH is due to the protonation of the amine moiety, which diminished the ability of the amine group to involve in chelate formation with Cd(II) and Cu(II). At pH > 6, precipitation of the metal hydroxide occurred, and therefore the absorbance of the analytes decreased as the sample pH increased. For further experiments, a pH range of 3.6-4.0 was used for the preconcentration.

Figure 4. Effect of pH on the on-line preconcentration of 4g L-1 Cd(II) and 10g L-1 Cu(II). All other conditions as in Table 1.
The effect of sample loading time on the preconcentration of 0.5 m g L-1 Cd(II) and 1 m g L-1 Cu(II) was tested at a sample loading flow rate of 9.6 mL min-1. It was found that the absorbance increased almost linearly as sample loading time up to 240 s. Studies on the effect of sample loading flow rate on the preconcentration of 4 m g L-1 Cd(II) and 10 m g L-1 Cu(II) for 30 s showed that the absorbance increased linearly as sample loading flow rate increased up to 11.6 mL min-1. These results indicated that the kinetics for the adsorption of Cu(II) and Cd(II) by the developed sorbent was fairly fast. The wide range of linearity shows the sorbent offered great potential application in flow injection on-line microcolumn preconcentration system for achieving high enhancement factors by increasing sample loading rates and/or sample loading time without losing retention efficiency.
Diluted hydrochloric acid was chosen for on-line elution of the retained analytes from the microcolumn packed with the AAP-GW. The effects of HCl concentration, its flow rate and elution time on the elution of the retained Cu(II) and Cd(II) was examined. It was found that 0.4 and 0.8 mol L-1 of HCl solution at a flow rate of 5.6 mL min-1 for 10 s elution of the retained Cd(II) and Cu(II) respectively was optimum for sensitivity and precision.
Once cadmium and copper elution were complete, the sorbent should be neutralized for the de-protonation of the amine moiety to recover the complex ability of the amine group. It was found that a 0.2 mol L-1 of ammonia solution at a flow rate of 5.6 mL min-1 applied for rinsing the microcolumn for 5 s after elution was quite efficient for this purpose.
The selectivity of the sorbent for on-line preconcentration of Cd(II) and Cu(II) was demonstrated by studying the effect of coexisting metal ions on the recovery of 4 m g L-1 Cd(II) and 10 m g L-1 Cu(II). As shown in Table 2, the presence of up to 150 mg L-1 of Na(I), 20 mg L-1 of Mg(II), 60 mg L-1 of Ca(II), 0.8 mg L-1 of Fe(III), 1 mg L-1 of Cr(III) and Zn(I), 2 mg L-1 of Ni(II), 1.5 mg L-1 of Pb(II), and 0.45 mg L-1 of Cu(II) had no significant interference with the on-line preconcentration of 4 m g L-1 Cd(II).
The tolerable concentrations of Na(I), Mg(II), Ca(II), Fe(III), Cr(III), Zn(II), Ni(II), Pb(II), and Cd(II) for the on-line preconcentration of 10 m g L-1 Cu(II) were found to be 600, 50, 100, 3, 1, 1.5, 4, 3 and 0.4 mg L-1, respectively. As demonstrated later, the present system allowed the interference-free preconcentration of trace Pb and Cd in the environmental and biological samples studied.
Table 2.Effect of Coexisting Ions on the On-Line Preconcentration of 4 m g L-1 Cd(II) and 10 m g L-1 Cu(II)
| Coexisting ion | concentration / mg L-1 |
Cd(II) | concentration /mg L-1 |
Cu(II) | |||
| [M]/[Cd] | recovery (%) | [M]/[Cu] | recovery (%) | ||||
| Na(I) | 80 | 20 000 | 99 ± 3 | 400 | 40 000 | 99 ± 4 | |
| 100 | 25 000 | 92 ± 5 | 600 | 60 000 | 91 ± 3 | ||
| 150 | 37 500 | 84 ± 2 | 800 | 80 000 | 80 ± 2 | ||
| Mg(II) | 10 | 2 500 | 98 ± 1 | 40 | 4 000 | 96 ± 3 | |
| 20 | 5 000 | 91 ± 2 | 50 | 5 000 | 88 ± 4 | ||
| 30 | 7 500 | 77 ± 3 | 60 | 6 000 | 78 ± 2 | ||
| Ca(II) | 50 | 12 500 | 99 ± 3 | 80 | 8 000 | 98 ± 2 | |
| 60 | 15 000 | 93 ± 2 | 100 | 10 000 | 91 ± 2 | ||
| 80 | 20 000 | 72 ± 2 | 120 | 12 000 | 82 ± 2 | ||
| Fe(III) | 0.5 | 125 | 96 ± 3 | 200 | 94 ± 3 | ||
| 0.8 | 200 | 87 ± 2 | 300 | 85 ± 5 | |||
| 250 | 79 ± 1 | 600 | 71 ± 6 | ||||
| Fe(II) | 0.5 | 125 | 98 ± 2 | 100 | 98 ± 2 | ||
| 250 | 91 ± 2 | 1.5 | 150 | 90 ± 2 | |||
| 500 | 73 ± 4 | 200 | 79 ± 3 | ||||
| Zn(II) | 0.5 | 125 | 98 ± 3 | 100 | 102 ± 3 | ||
| 250 | 96 ± 1 | 1.5 | 150 | 93 ± 4 | |||
| 500 | 82 ± 1 | 200 | 83 ± 2 | ||||
| Cr(III) | 0.8 | 200 | 101 ± 1 | 0.8 | 80 | 97 ± 3 | |
| 250 | 94 ± 2 | 100 | 84 ± 4 | ||||
| 1.5 | 375 | 83 ± 3 | 1.5 | 150 | 73 ± 4 | ||
| Ni(II) | 250 | 98 ± 2 | 200 | 96 ± 4 | |||
| 500 | 89 ± 2 | 400 | 87 ± 3 | ||||
| 1 250 | 77 ± 1 | 800 | 78 ± 2 | ||||
| Pb(II) | 250 | 99 ± 3 | 200 | 98 ± 3 | |||
| 1.2 | 300 | 92 ± 4 | 2.5 | 250 | 90 ± 4 | ||
| 1.5 | 375 | 86 ± 2 | 300 | 86 ± 2 | |||
| Cu(II) | 0.2 | 50 | 101 ± 2 | ||||
| 0.4 | 100 | 98 ± 1 | |||||
| 0.45 | 113 | 86 ± 3 | |||||
| Cd(II) | 0.2 | 20 | 98 ± 2 | ||||
| 0.4 | 40 | 90 ± 3 | |||||
| 0.5 | 50 | 76 ± 4 |
3.4 Figures of Merit for the Application of the AAP-GW Sorbent in FI On-Line Microcolumn Preconcentration and Separation FAAS
Table 3. Analytical Performance of the FI On-line Microcolumn Preconcentration Coupled with FAAS for Determination of Trace Cd(II) and Cu(II)
| Cd | Cu | |
| preconcentration time/s | 30 | 30 |
| enhancement factor | 124 | 61 |
| sampling frequency/h | 80 | 80 |
| sample consumption/mL | 4.8 | 4.8 |
| eluent consumption/mL regeneration reagent consumption/mL |
0.93 0.47 |
0.93 0.47 |
| precision (RSD, = 11)% | 0.8 (4 m g L-1 | 0.5 (10 m g L-1 |
| detection limit (3s )/ng L-1 | 14 | 31 |
| range of calibration graph/m g L-1 | 0.5-30 | 2-40 |
| calibration function (N = 6) , absorbance, in m g L-1 |
= 0.022C + 0.0095 | = 0.0097C + 0.0042 |
| correlation coefficient | 0.9995 | 0.9998 |
The analytical performance characteristic data of the FI on-line microcolumn preconcentration and separation using the AAP-GW sorbent coupled with FAAS for determination of trace Cd(II) and Cu(II) were given in Table 3. With consumption of 4.8 mL of sample solution, the enrichment factor (EF) obtained by comparing the slopes of the linear portion of the calibration curves before and after the preconcentration was 124 and 61 for Cd(II) and Cu(II), respectively at a sampling frequency of 80 h-1 (calibration function (N = 6) (, absorbance, in m g L-1) Cd = 0.022 + 0.0095; Cu = 0.0097 + 0.0042, Cd and Cu direct aspiration calibration Cd = 1.77 ´ 10-4 – 0.0029; Cu = 1.59 ´ 10-4 – 0.0021, EFCd = 0.022 / 1.77 ´ 10-4 = 124; EFCu = 0.0097 / 1.59 ´ 10-4 = 61).The concentration efficiency (CE), defined as the product of EF and the sampling frequency, was 248 and 123 EF min-1for Cd(II) and Cu(II), respectively. The retention efficiency, i.e. the percentage of sample collected on the microcolumn, was 93% and 92%, respectively for Cd(II) and Cu(II), indicating that the AAP-GW packed microcolumn had an adequate capacity and a fast retention kinetics even at high sample loading flow rates. The precision (RSD) for 11 replicate preconcentrations of 4 m g L-1 Cd(II) and 10 m g L-1 Cu(II) was 0.8 and 0.5%, respectively. The new sorbent also offered good linearity (g >0.9995) for on-line preconcentration of trace Cd(II) and Cu(II).
3.5 Validation of the Developed FI On-Line Microcolumn Preconcentration and Separation FAAS System Using the AAP-GW Sorbent
A number of certified reference materials (CRMs), GBW 08607 (River Water), GBW 07601 (Human Hair) and GBW07605 (Tea), were analyzed to evaluate the accuracy of the AAP-GW sorbent-based FI on-line microcolumn preconcentration and separation coupled with FAAS. As shown in Table 4, the concentrations of Cd and Cu in these CRMs obtained by the present method using simple aqueous standard calibration agreed well with the certified values, demonstrating the applicability of the developed FI on-line microcolumn preconcentration system coupled with FAAS for the determination of trace Cd and Cu in the environmental and biological samples studied.
Table 4. Analytical Results (m g g-1, mean ±σ, n = 3) for the Determination of Trace Cadmium and Copper in the Certified Reference Materials (CRMs)
| sample | concentration of Cd | concentration of Cu | |||
| certified | determined | certified | determined | ||
| GBW 08607 (river water) |
0.100 ± 0.02 | 0.095 ± 0.02 | 1.00 ± 0.01 | 0.974 ± 0.03 | |
| GBW 07601 (human hair) |
0.11 ± 0.03 | 0.10 ± 0.02 | 10.6 ± 1.21 | 10.1 ± 1.44 | |
| GBW 07605 (tea) |
0.057 ± 0.010 | 0.048 ± 0.02 | 17.3 ± 1.82 | 15.5 ± 2.23 |
4. CONCLUSION
A new diamine functionalized glass fibre sorbent was prepared for preconcentration and separation of toxic metal ions from aqueous solution by loading tetraethoxysilicate (TEOS) and N-(2-aminoethyl)-3-aminopropyltrimethoxysilane (AAPTS) on glass fibre through a sol-gel process. The new sorbent provided good acid-base stability, fast adsorption and desorption kinetics, and good linearity, making the material very suitable for its application in FI on-line microcolumn preconcentration and separation systems.
Acknowledgements
This work was supported by National Science Foundation of China (No. 20675024), Natural Science Foundation of Hebei Educational Committee (No. 2006407), China Postdoctoral Science Foundation (No. 2005037629) and Science Foundation of Hebei University.
