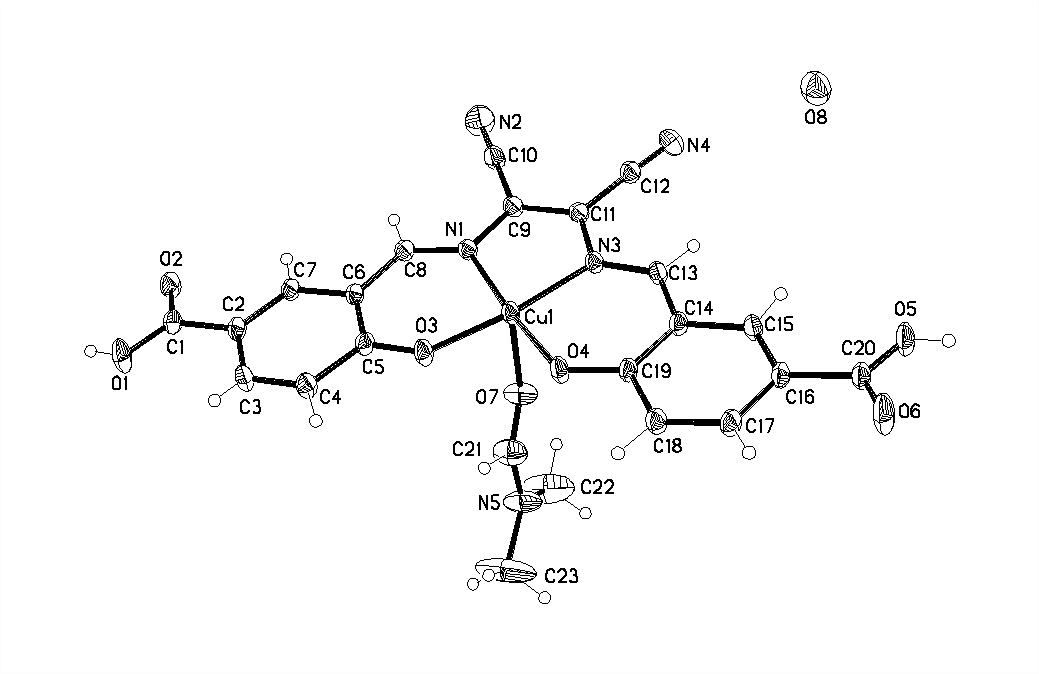
Abstract The Salen-copper(II) complex was synthesized by diffusion. Chemistry Online shows the structure which was characterized by single crystal X-ray diffraction. It crystallized in triclinic system,pB1spacegroup, with a=9.3441(18)Å, b=11.478(3)Å, c=12.320(3)Å, a=81.584(11)°, b=74.681(9)°, g=68.206(9), MolecularFormula: C23H19CuN5O8, M=397.86, V=1181.6(5)Å3, z=2, Dc=1.56Mg/m3, R1=0.0416, wR2=0.1236,I>[2s/(I)]. The crystal included a mononuclear complex [Cu(H2L)(DMF)] and a non-coordinated H2O molecular.
Keyword diaminomaleontrile;5-carboxylsalicylaldehyde;crystal structure.
Synthesis and Crystal Structure of a New Salen-Copper (II) Complex
Li Lijun, Yang Dongjie, Gao Yansu
(College of Chemistry and Environmental Science, Hebei University, Baoding, Hebei 071002, China )
Summary diffusion were synthesized Salen- copper (Ⅱ) mononuclear complexes [a Cu (H 2 L) (of DMF)] . H 2 0 [H . 4 L] = N, N’-bis (5-carboxy-salicylaldehyde) – Diaminomaleonitrile. The complexes were characterized by X-ray single crystal diffraction. The complexes belong to triclinic system, …